Background: Pain associated with third molar surgery significantly impacts activities of daily living for approximately 1–2 days.1 Infiltration of local anesthetics into a surgical site is an effective analgesic technique that is associated with low risk of systemic adverse events (AEs).2 Approved by the FDA in 2011, EXPAREL (liposomal bupivacaine, [LB]) is a prolonged-release formulation of bupivacaine used for surgical site infiltration, and has been used for postsurgical analgesia in over 1.5 million patients undergoing various surgical procedures. In this study, LB was evaluated in subjects undergoing bilateral maxillary and mandibular third molar removal, with ≥1 mandibular third molar fully or partially bony impacted. The primary objective was to evaluate the analgesic efficacy of LB following third molar surgery.
Materials and Methods: Following IRB approval and written consent, adult subjects were enrolled in this prospective, randomized, double-blind, placebo-controlled study. Subjects were randomized to receive LB (133 mg/10 mL) or placebo (10 mL saline). At the start of surgery, randomized subjects received a nerve block (lidocaine 2% with epinephrine 1:100,000). At least 20 minutes after nerve block, LB or saline was infiltrated into all 4 surgical sites. Following the procedure, subjects received rescue oral oxycodone 5–10 mg q4h upon request. A 0- to 10-point numeric rating scale (NRS) measured pain intensity. The primary endpoint was to assess analgesic efficacy by determining area under the curve (AUC) of NRS scores through 48h. Secondary endpoints measuring analgesia included AUC of the NRS scores through 24h, 72h, and 96h. Opioid utilization through 48h (morphine equivalent dose [MED], mg), time to first rescue, and subject satisfaction were also assessed. AEs through Day 30 and vital signs immediately before and following the procedure were recorded for all subjects who were randomized and treated. Analysis of variance (ANOVA) interaction model was used for statistical evaluation of the analgesic efficacy endpoints.
Results: A total of 166 subjects were enrolled in the study. Eighty-nine subjects (LB=59; placebo=30) were included in this analysis, and 77 subjects were excluded due to protocol deviations. For the primary endpoint, AUC of NRS scores at 48h, the LB group least-squares (LS) mean score (LS mean [SEM]= 120.8 [13.9]) was significantly lower (P=0.0226) than placebo group scores (183.3 [23.0]) (Figure 1). Pain scores were also significantly lower in the LB group at 24h (LB=77.9 [6.8] vs placebo=108.9 [11.1], P=0.0192), 72h (LB=151.5 [20.0] vs placebo=229.2 [32.9] P=0.0469), and 96h (LB=173.3 [24.7] vs placebo=270.2 [40.7], P=0.0450) (Figure 1).
Figure 1. Postoperative Pain Levels: EXPAREL vs Placebo.
Patients in the LB group (LS mean [SEM]=2.9 [1.2]) required fewer opioids (P=NS) than patients in the placebo group (3.22 [1.3]). Median [95% CI] time to first rescue was similar between groups (LB, 4.3 mg [4.1, 6.1] vs placebo 3.4 mg [2.8, NE]), as was subject satisfaction at 24–96h. There were no differences in AE rates between groups, and treatment-emergent AEs were mild or moderate.
Conclusions: In this preliminary study, LB provided prolonged postsurgical analgesia compared to placebo, and this difference was statistically significant from 24h through 96h. The safety profile for LB did not differ from placebo and was consistent with previously reported safety profiles for LB. These preliminary results demonstrate that further studies are warranted to investigate the potential of LB for prolonged pain management following oral surgery.
References
1. Bienstock DA, Dodson TB, Perrott DH, Chuang SK. J Oral Maxillofac Surg. 2011;69(5):1272-1277.
2. Joshi GP, Beck DE, Emerson RH, et al. Am Surg. 2014;80(3):219-228.



 Indicates sessions with Audience Response Systems (ARS) or Poll Everywhere available
Indicates sessions with Audience Response Systems (ARS) or Poll Everywhere available Indicates sessions that require a ticket for attendance
Indicates sessions that require a ticket for attendance Indicates Practice Management and Allied Staff Sessions Day Pass.
Indicates Practice Management and Allied Staff Sessions Day Pass.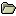 Indicates sessions with recordings available for purchase.
Indicates sessions with recordings available for purchase.